Developing a robust crystallization process to isolate the optimal solid form of an API is an essential step in API development. Identifying the crystallization approach, solvent system(s), controlling form, crystal habit and delivering a consistent uniform PSD from a single process step can be very challenging.
The key to controlled, growth-dominated crystallization is a thorough understanding of the solubility and metastable zone width (MSZW) of the system in combination with knowledge of the nucleation and growth kinetics. Once this understanding is acquired by experimental techniques, PAT and modeling, the process can be optimized to target crystal growth and avoid unwanted nucleation events (route 2).
 Pictorial representation of different crystallization pathways with respect to solubility and MSZW
Pictorial representation of different crystallization pathways with respect to solubility and MSZW
The arrival of advanced high-resolution PAT such as the BlazeMetrics™ probe gives previously unattainable insights into both existing and novel crystallization processes. A combination of high-resolution microscopy and Raman spectroscopy allows the formation and growth of transient forms to be pinpointed and the materials identified in situ.
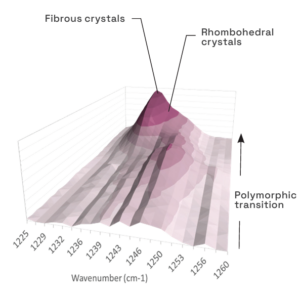
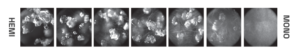
In situ Raman spectroscopy and imaging of a polymorphic transition from a metastable form with rhombohedral morphology to a stable form with fibrous morphology.
Through advanced characterization tools, our experienced team of scientists are able to examine crystallization systems at reduced scales. Chord length measurements and in situ images can be used to validate crystal growth models at a small scale. Breakage, aggregation, and secondary nucleation can all be assessed throughout the scale-up process and these events are factored into predictive models during method transfer from vessel to vessel.
Our experts are trained not only in the art of crystallization, but also in solid form science, modeling, PAT, and particle sizing.
